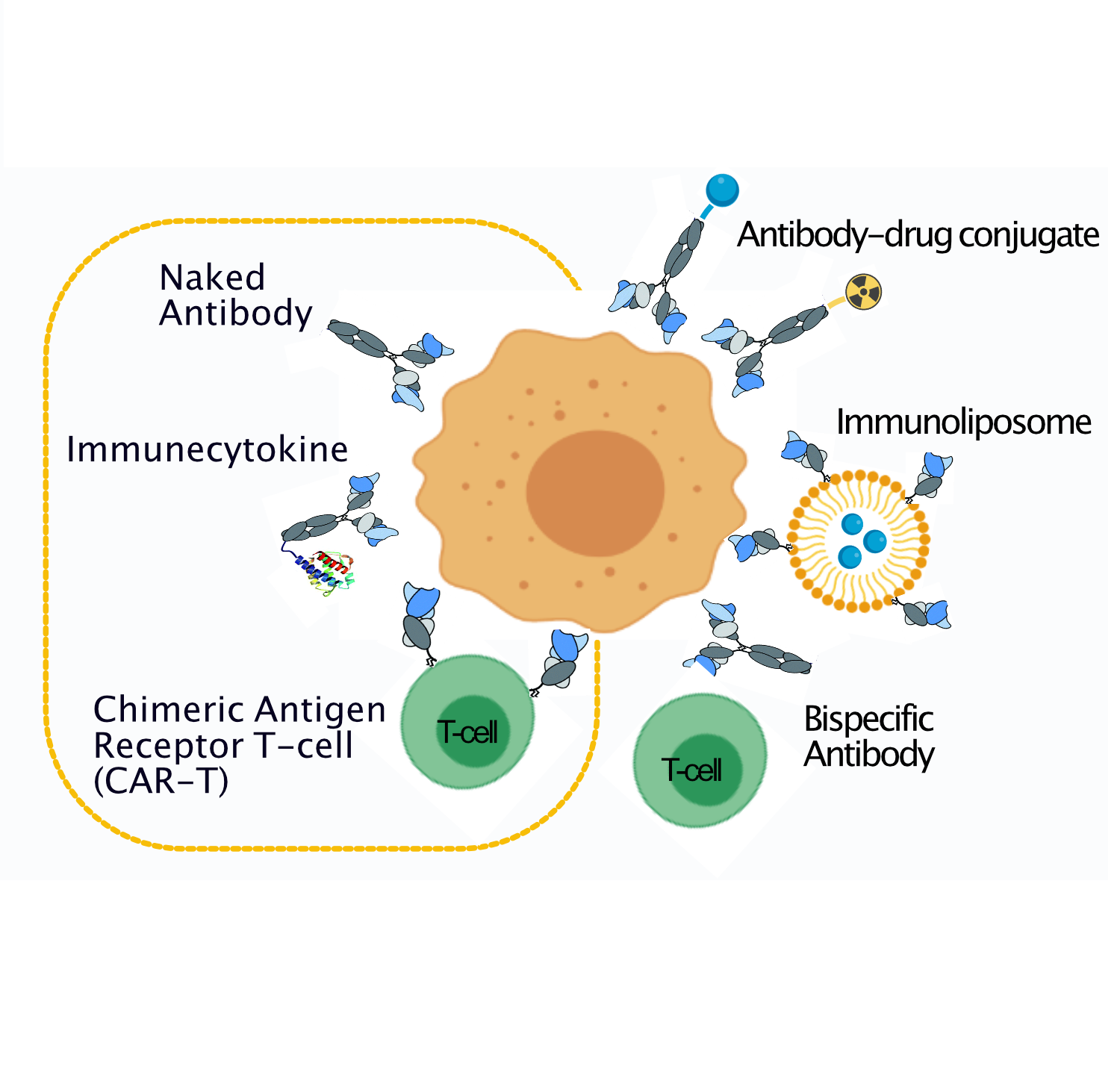
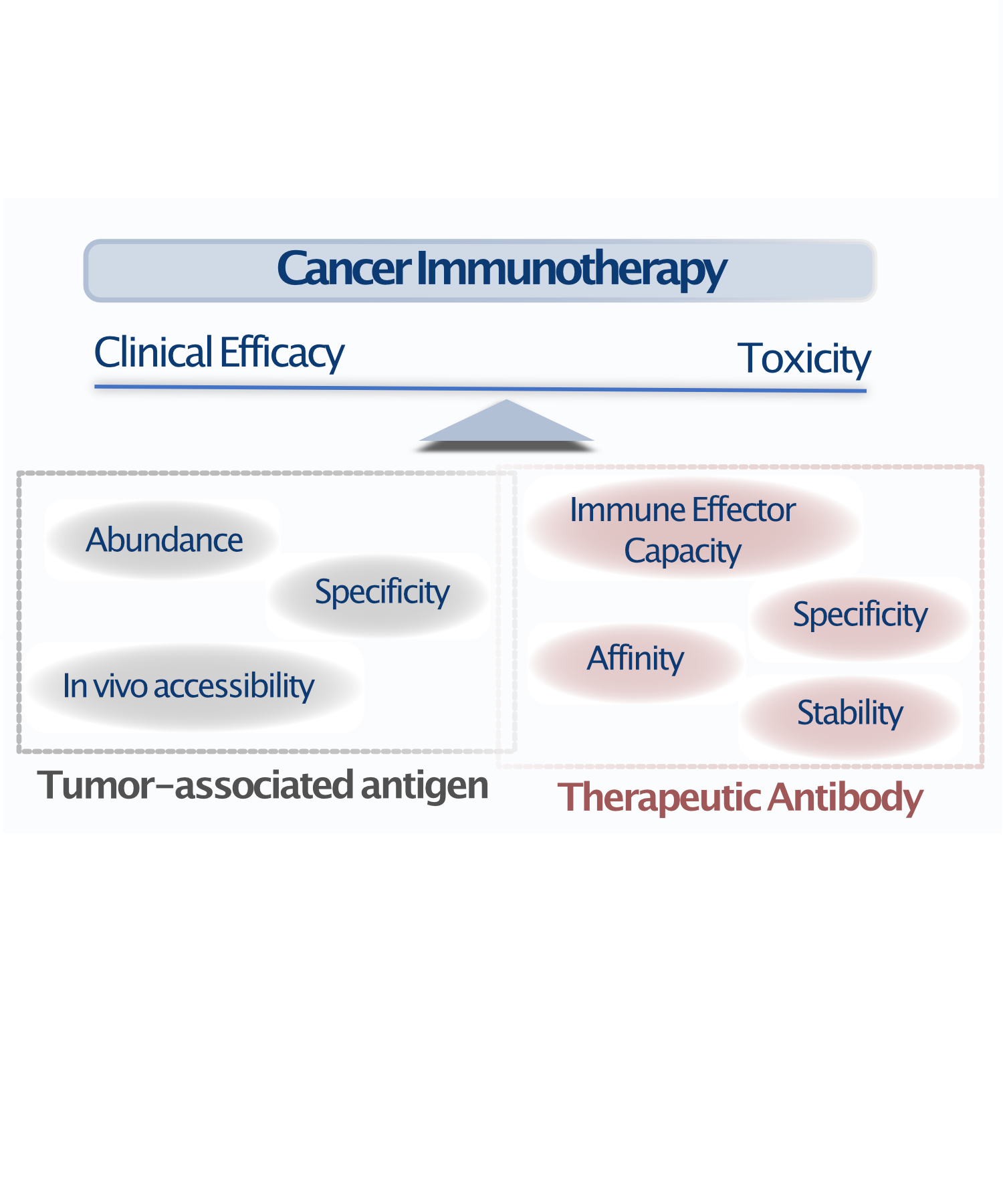
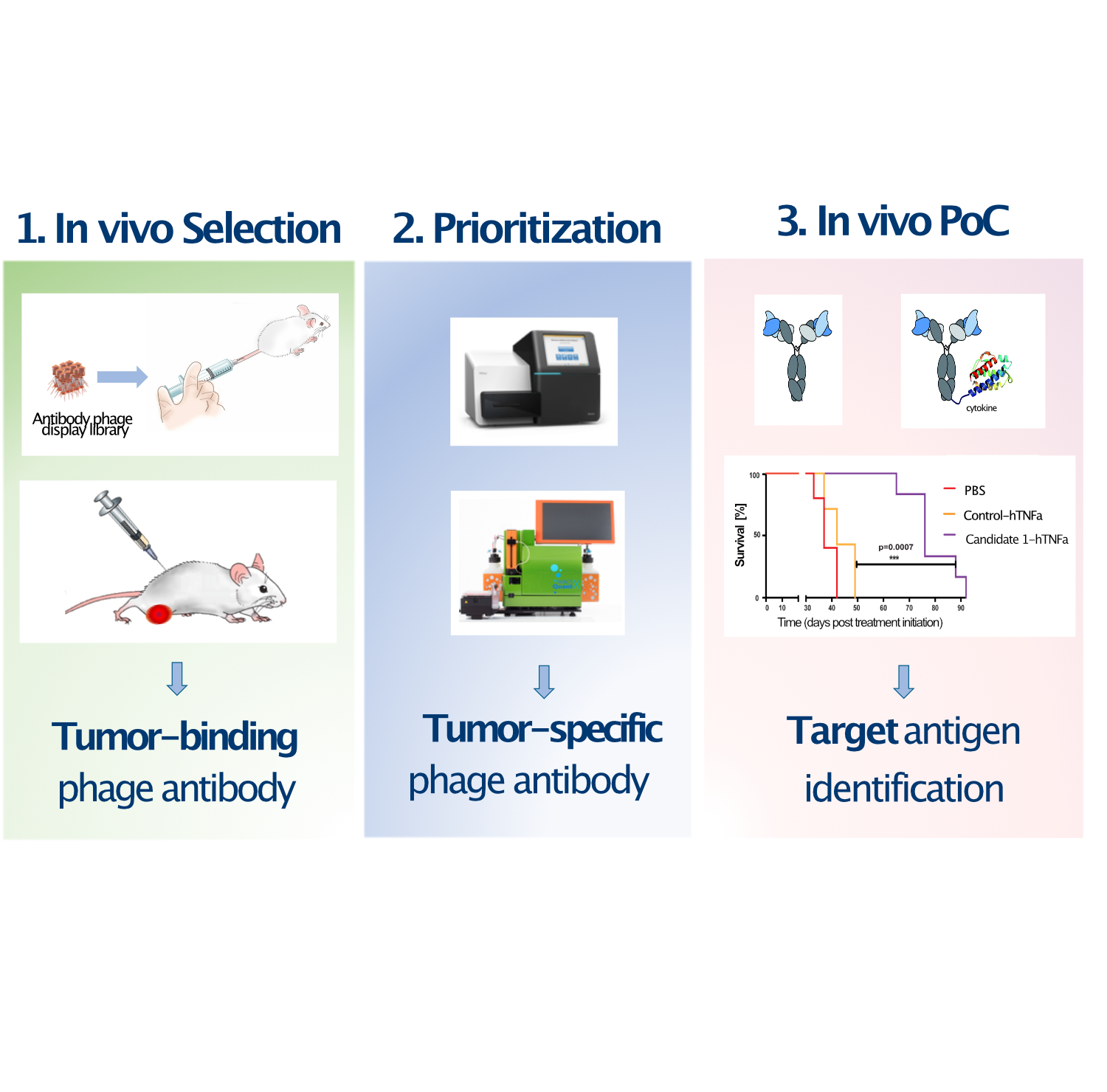
Since the approval of the first monoclonal antibody “rituximab”, immunotherapies have dramatically changed the natural history of several cancer types. However, due to the paucity of viable tumor antigens discovered by the standard target-first approach, only few antibody-based drugs have entered into clinical use, being able to help only a fraction of all cancer patients.